Examples of Effusion In Daily Life – Effusion is the movement of a gas or liquid through a small opening or porous material. This phenomenon occurs in many aspects of daily life but is often overlooked or taken for granted. By understanding the concept of effusion, we can better appreciate and understand the world around us.
Graham’s Law of Effusion
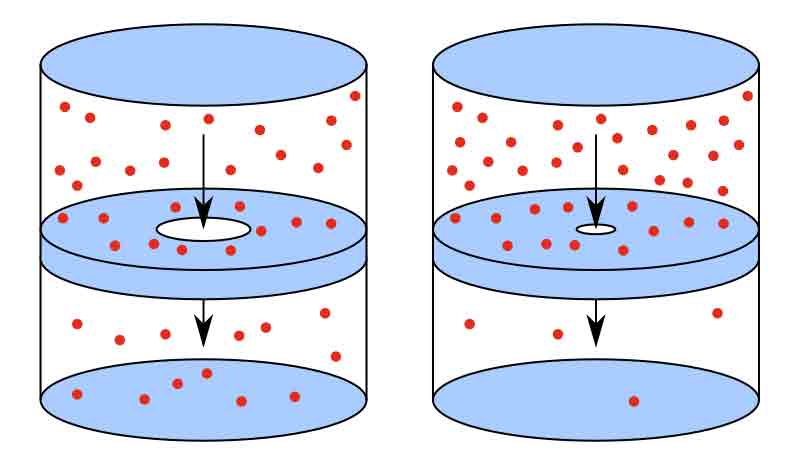
Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This means that the lighter the gas, the more quickly it will effuse through a small opening or porous material. The equation for Graham’s Law of Effusion is:
Rate of effusion (A) / Rate of effusion (B) = (Square root of molar mass (B)) / (Square root of molar mass (A))
This law was first discovered by Thomas Graham in 1829, and it is based on the kinetic theory of gases, which states that gases consist of particles in constant random motion. According to this theory, the lighter the gas, the more rapidly its particles will move, which will allow them to effuse through a small opening or porous material more quickly.
Graham’s Law of Effusion is used in many fields such as chemistry, physics, and biology to calculate the rate of effusion of gases. It is also used in gas chromatography to separate the individual components of a sample based on their rate of effusion, and in mass, spectroscopy to determine the mass of molecules by measuring the rate of effusion of the molecules through the small opening in the mass spectrometer.
In summary, Graham’s Law of Effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This law was first discovered by Thomas Graham in 1829, and it is based on the kinetic theory of gases. It is used in many fields such as chemistry, physics, and biology, and it is important for the understanding of effusion in gases.
Examples of Effusion In Daily Life
In this article, we will explore some real-life examples of effusion in cooking, tire pressure, medicine, and plants, chemistry, and understand how it plays a role in our everyday lives.
Effusion in cooking
Effusion in cooking refers to the movement of a liquid or gas through a small opening or porous material. An example of effusion in cooking is when oil is heated in a pan. As the oil is heated, the molecules of the oil move more rapidly and start to move through the small openings on the surface of the pan. This causes the oil to spread out in the pan, which is an example of effusion.
Another example of effusion in cooking is when boiling water in a pot. As the water is heated, the water molecules start to move more rapidly and start to move through the small opening in the surface of the pot, which causes the water to start boiling, and the steam to escape through the lid.
Effusion is also important in cooking when it comes to the release of gases and aromas from food. For example, when meat is cooked, the heat causes the fat and juices inside the meat to move more rapidly. This causes the fat and juices to effuse through the small openings in the surface of the meat, which results in the release of flavorful gases and aromas.
Effusion is also seen when grilling food, especially when the food is marinated with a liquid that contains volatile compounds. When the food is grilled, the heat causes the marinade to effuse through the small openings in the surface of the food, which results in the release of flavorful gases and aromas.
In summary, Effusion is an important concept in cooking that can be seen in different forms such as oil spreading in a pan, boiling water, the release of gases and aromas, and more. Understanding the concept of effusion can help cooks better understand how heat and temperature affect the food they are cooking, and how to control and manipulate the flavors of the food.
Effusion in tire pressure
Effusion in tire pressure refers to the movement of air out of a tire through a small opening or puncture. When a tire is properly inflated, the air molecules inside the tire are under pressure, and they are held in place by the walls of the tire. However, when a tire becomes punctured, the air molecules escape through the small opening caused by the puncture. This results in a decrease in tire pressure, which is an example of effusion.
Effusion in tire pressure can be caused by a variety of factors such as road hazards such as nails, glass, or other sharp objects, improper tire maintenance, or simply through the normal wear and tear of a tire. It is important to regularly check tire pressure and inspect tires for any punctures or damage to prevent air from escaping and avoid a flat tire.
Effusion in tire pressure can also be caused by a slow leak, which is a small puncture that doesn’t cause the tire to go flat immediately but rather causes the tire pressure to drop gradually. Slow leaks are often harder to detect and can cause serious problems if left unchecked.
Preventing effusion in tire pressure is important for maintaining the safety and efficiency of a vehicle. Proper tire maintenance, regular tire pressure checks, and regular tire inspections are essential to prevent effusion and ensure that the tires are in good working condition.
In summary, Effusion in tire pressure is the movement of air out of a tire through a small opening or puncture, which causes a decrease in tire pressure. It can be caused by road hazards, improper tire maintenance, or normal wear and tear, and it is important to regularly check tire pressure and inspect tires to prevent effusion and ensure the safety and efficiency of a vehicle.
Effusion in medicine
In medicine, effusion refers to the buildup of fluid in a body cavity. One common example of effusion in medicine is pleural effusion, which occurs when there is an excess of fluid in the pleural cavity, the space between the lung and the chest wall. This can be caused by a variety of conditions such as pneumonia, heart failure, cancer, or injury, and it can cause difficulty in breathing.
Another example of effusion in medicine is peritoneal effusion, which occurs when there is an excess of fluid in the peritoneal cavity, the space that surrounds the organs in the abdomen. This can be caused by conditions such as liver disease, kidney disease, or cancer.
Effusion can also occur in the joints, such as in the knee, shoulder, or elbow, which is known as joint effusion. This can be caused by conditions such as osteoarthritis, rheumatoid arthritis, or injury, and it can cause pain and discomfort.
Effusion in medicine can be diagnosed by a variety of methods such as chest X-ray, CT scan, ultrasound, or by inserting a needle into the affected area and removing some of the fluid for analysis. Treatment for effusion will depend on the underlying cause and can include draining the fluid, administering medication, or surgery.
In summary, Effusion in medicine refers to the buildup of fluid in a body cavity. It can occur in the pleural cavity, the peritoneal cavity, or joints, and it can be caused by a variety of conditions such as pneumonia, heart failure, cancer, or injury. Effusion can be diagnosed by a variety of methods and treatment will depend on the underlying cause.
Effusion in plants
Effusion in plants refers to the movement of fluids such as sap through small openings or porous materials in the plant. One example of effusion in plants is the flow of sap through tree branches. The sap is under pressure in the tree’s trunk, and it flows through small openings in the branches to reach the leaves. This process is essential for the tree’s survival and growth, as the sap carries nutrients and water to the leaves, where photosynthesis occurs.
Another example of effusion in plants is the movement of water through the roots and stem of a plant. The water is under pressure in the soil, and it flows through small openings in the roots and stems to reach the leaves and other parts of the plant. This process is essential for the plant’s survival as it provides the plant with the water and nutrients it needs to grow.
Effusion in plants is also seen when plants release gases and volatile compounds, such as essential oils. These compounds are stored in the plant’s cells and are released through small openings in the plant’s surface. This process is important for the plant’s survival, as it can help the plant to attract pollinators, repel predators, or adapt to environmental changes.
In summary, Effusion in plants refers to the movement of fluids such as sap, and water through small openings or porous materials in the plant. It is essential for the plant’s survival and growth, as it allows the plant to transport nutrients, water, and gases. Understanding the concept of effusion in plants can help us better appreciate and understand the natural world around us.
Effusion in the atmosphere
Effusion in the atmosphere refers to the movement of air from a high-pressure area to a low-pressure area. This process is driven by the differences in air pressure between the two areas, with the air molecules in the high-pressure area being more densely packed than in the low-pressure area. As a result, the air molecules tend to flow out of the high-pressure area and into the low-pressure area through openings or gaps such as passes, valleys, or coastlines.
One example of effusion in the atmosphere is wind. The wind is caused by the movement of air from high-pressure areas to low-pressure areas. As the air molecules in the high-pressure area flow out of the area and into the low-pressure area, they create a wind. This process is driven by the sun’s heating of the earth’s surface, which causes differences in air pressure.
Another example of effusion in the atmosphere is the formation of clouds. Clouds form when the air in the atmosphere rises and cools, causing the water vapor in the air to condense into tiny droplets or ice crystals. This process is driven by the effusion of air from high-pressure areas to low-pressure areas.
Effusion in the atmosphere also plays a role in the formation of storms such as thunderstorms, hurricanes, and tornadoes. These storms form when air rises rapidly and cools, causing the water vapor in the air to condense into clouds and precipitation. This process is driven by the effusion of air from high-pressure areas to low-pressure areas.
In summary, Effusion in the atmosphere refers to the movement of air from high-pressure areas to low-pressure areas. This process is driven by the differences in air pressure between the two areas, and it plays a role in the formation of wind, clouds, and storms such as thunderstorms, hurricanes, and tornadoes. Understanding the concept of effusion in the atmosphere can help us better understand and appreciate the weather and the natural world around us.
Related: Impact of Human Interaction with the Natural Environment
Effusion in chemistry
In chemistry, effusion is the process of a gas or vapor passing through a small opening or porous material. This phenomenon is important to understand in many chemical reactions and processes.
One example of effusion in chemistry is the use of effusion cells in determining the molar mass of a gas. Effusion cells consist of a small opening through which a gas can pass, and a second compartment into which the gas effuses. By measuring the rate of effusion, the molar mass of the gas can be calculated.
Another example is the use of effusion in gas chromatography. In gas chromatography, a sample is vaporized and separated into its components as it passes through a small opening or porous material. The rate of effusion of each component through the opening is used to separate and identify the individual components of the sample.
In addition to that, effusion is also used in Mass spectroscopy to measure the mass of molecules. In this process, molecules are ionized, and then they are passed through small openings in the mass spectrometer. The rate of effusion of the molecules through the opening is used to determine their mass.
Effusion is also used in the field of thermodynamics. Effusion is important in understanding how gases behave under different conditions of pressure and temperature. For example, the ideal gas law is based on the idea that the molecules of a gas are in constant random motion, and that they effuse through small openings or porous materials.
Overall, effusion is a fundamental concept in chemistry that plays a role in many chemical reactions and processes. Understanding effusion can help us better understand and appreciate the chemical world around us.
Related: Sources of Thermal Energy on Earth
Effusion in physics
Effusion in physics refers to the movement of particles through a small opening or porous material. This phenomenon is important to understand in many physical systems and processes.
One example of effusion in physics is the effusion of gases through small openings. The kinetic theory of gases states that gases consist of particles in constant random motion. These particles will naturally tend to move from a region of high concentration to a region of low concentration, and this process is known as effusion.
Another example of effusion in physics is the effusion of particles through porous materials. In this case, the particles move through the small openings or pores in the material, and the rate of effusion is related to the size and number of openings or pores.
Effusion is also important in the study of thermodynamics. Effusion is related to the movement of particles in a system and it is an important concept in understanding the behavior of gases under different conditions of pressure and temperature.
In physics, effusion is also related to the study of diffusion, which is the process of the spread of matter or energy from a higher concentration to a lower concentration. Effusion and diffusion are related concepts that are present in many physical systems and processes.
In summary, Effusion in physics refers to the movement of particles through a small opening or porous material, which is important to understand in many physical systems and processes. It is related to the study of gases, thermodynamics, and diffusion. Understanding the concept of effusion in physics can help us better understand and appreciate the physical world around us.
Effusion in mechanics
Effusion in mechanics refers to the movement of a fluid or gas through a small opening or porous material in mechanical systems. This phenomenon plays a role in many mechanical systems and processes.
One example of effusion in mechanics is the movement of air or fluid through a porous filter. The filter is designed to allow the fluid or air to pass through small openings while trapping unwanted particles. This process is used in many mechanical systems such as air conditioning, heating, and filtration systems.
Another example of effusion in mechanics is the movement of fluids through small openings in seals or gaskets. These seals and gaskets are designed to prevent the leakage of fluids in mechanical systems such as pumps, engines, and gearboxes.
Effusion also plays a role in the study of porous materials such as ceramics and metal foams. These materials have small openings or pores that allow fluids or gases to pass through, which is an important property for many mechanical applications such as heat exchangers, catalysts, and sound absorbers.
In summary, Effusion in mechanics refers to the movement of a fluid or gas through a small opening or porous material in mechanical systems. It plays a role in many mechanical systems and processes such as filters, seals, porous materials, and more. Understanding the concept of effusion in mechanics can help us better understand and appreciate the mechanical systems and processes that are present in our everyday lives.
Related: Properties of Solids, Liquids and Gases
Effusion in biology
Effusion in biology refers to the movement of molecules or particles through small openings or pores in biological systems. This phenomenon plays a role in many biological processes and systems.
One example of effusion in biology is the movement of gases such as oxygen and carbon dioxide through the small openings in the surface of leaves. In plants, these small openings are called stomata, and they allow gases to move in and out of the leaf, which is important for photosynthesis, the process by which plants convert light energy into chemical energy.
Another example of effusion in biology is the movement of ions and small molecules through the cell membrane. The cell membrane is a selectively permeable membrane that surrounds the cell and it allows certain molecules or ions to pass through while excluding others. This process is known as facilitated diffusion and it is essential for the survival of cells.
Effusion also plays a role in the study of osmosis, which is the movement of water molecules through a selectively permeable membrane. Osmosis is important for the survival of cells and organisms as it helps to maintain the balance of water and solutes within the cell.
In summary, Effusion in biology refers to the movement of molecules or particles through small openings or pores in biological systems. It plays a role in many biological processes and systems such as photosynthesis, facilitated diffusion, and osmosis. Understanding the concept of effusion in biology can help us better understand and appreciate the living world around us.
Related: Examples of Thermal Expansion in Everyday Life
Conclusion
In conclusion, effusion is a common and often overlooked phenomenon that plays a significant role in many aspects of daily life. From cooking and tire pressure to medicine and plants, effusion can be observed in a wide range of contexts.
Understanding the concept of effusion can help us better appreciate and understand the world around us. It is a fundamental concept that is present in many fields, including physics, chemistry, mechanics, and more.
The next time you cook a meal, change a tire or observe the leaves of a tree, take a moment to consider the effusion that is taking place. Overall, Effusion is an integral part of our daily life, and understanding it can help us lead a better and more efficient life.
Well, those are some examples of effusion in daily life. Hope this helps!